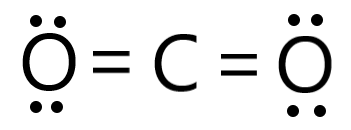It naturally occurs in the Earth’s atmosphere and plays an active role as a greenhouse gas. It is also held responsible for global warming and acid rain. It is released in the atmosphere as a product of many natural processes such as combustion, fermentation, etc. It is used by plants for the purpose of photosynthesis. Industrially, carbon dioxide is used in fire extinguishers, life jackets, refrigerants, etc. Many of you may have this question that is co2 organic or inorganic substance? In this article, we will study the same. Carbon dioxide is an inorganic compound. A compound is said to be organic if it contains a carbon atom bonded with a hydrogen atom. However, in the case of carbon dioxide, one atom of carbon is bonded with two atoms of oxygen i.e. no hydrogen atoms are present in this molecule. This indicates that carbon dioxide is not an organic compound. Well, this is not as simple as that. There exist many exceptions in chemistry. Better to check out the complete article for detailed information about whether CO2 is inorganic and the reason behind it.
Why is CO2 Inorganic?
Generally, the compounds in which two or more atoms other than carbon are bonded to form a molecule or those that lack carbon atom(s) as one of the constituents of their molecules are termed as the inorganic compounds. Also, most inorganic compounds consist of one or more metal atoms, are soluble in water, are non-flammable, and have high melting and boiling points. Carbon dioxide is formed by two atoms of oxygen bonded with one carbon atom. A compound is said to be organic when it contains one or more carbon atoms bonded with one or more hydrogen atoms. As there are no hydrogen atoms present in the carbon dioxide molecule to bond with the available carbon atom, it is an inorganic compound. Also, carbon dioxide molecule exhibits other properties of inorganic compounds such as it is non-flammable in nature rather it is used in fire extinguishers to douse the fire, it dissolves in water to form carbonic acid, etc.
Organic Vs Inorganic Compounds — Detailed Comparison
Historically, the compounds that derived from any living organism (which may be dead now) were called organic compounds. However, after conducting a number of researches it was concluded that this is not true and many inorganic compounds may also form parts of many living organisms. Therefore, the definition of organic compounds was changed to carbon-containing compounds. However, many carbon-containing inorganic compounds are known to occur that are found in nature and can also be synthesized artificially. Finally, it was agreed that the organic compounds were those that contained either carbon-carbon or carbon-hydrogen bonds while all other compounds were called inorganic. The main differences between organic and inorganic compounds are listed below: • Generally, the compounds containing carbon are classified as organic compounds. Similarly, the compounds not containing carbon are termed inorganic compounds. However, there are certain exceptions to this definition of inorganic compounds, such as carbon dioxide, carbon monoxide, carbonates, cyanides, etc. • Most organic compounds are formed by carbon atoms bonded with hydrogen, oxygen, nitrogen, chlorine, sulfur, and other elements. However, there are certain exceptions such as HCN is an inorganic compound even when it contains a carbon-hydrogen as well as carbon-nitrogen bond. • The organic compounds may exist in any form of matter such as solid, liquid, or gas. However, most inorganic compounds occur as solids with a few exceptions such as CO2, O2, NO2, NH3, etc. • The organic compounds are mostly insoluble in water while they are quite soluble in organic solvents such as benzene, toluene, etc. On the other hand, inorganic compounds are mostly soluble in water with a few exceptions such as carbonates, silicates, etc. • The organic compounds are formed due to covalent bonding between carbon and other atoms while in inorganic compounds the atoms may either be bonded through ionic or covalent bonds. • The organic compounds mostly do not conduct electricity when dissolved in water while the aqueous solutions of inorganic compounds are known to readily conduct electricity. • The organic compounds are mostly found in biological materials and are very complex in nature. However, the inorganic compounds mostly occur as minerals in nature and do not show much complexity. • There are mostly no corresponding salts for organic compounds while the inorganic compounds willingly form salts. However, there are still exceptions organic salts are formed when the hydrogen atoms in carboxylic acid are replaced by metal ions such as potassium, sodium, etc. • The reaction rate in organic compounds is quite slow compared to inorganic compounds, where the rate of the reaction is considerably high. • The melting and boiling points of organic compounds are relatively higher in comparison to the inorganic compounds. • The organic compounds are mostly volatile and inflammable in nature while the inorganic compounds are usually non-volatile and not inflammable. • A few examples of organic compounds are sugar, nucleic acids, hydrocarbons, enzymes, proteins, etc. While some examples of inorganic compounds are acids, bases, metals, salts, carbonates, etc.
Bonding in CO2 Molecule
Carbon belongs to group 14 and has 4 valence electrons, while oxygen is a group 16 element and has 6 electrons in its valence shell. This indicates that a carbon atom needs four electrons to complete its octet while the oxygen atom requires two electrons in its valence shell to become stable. Whenever a carbon and an oxygen atom are close, they bond together. When two oxygen and one carbon atom are available, they bond to form carbon dioxide. The carbon and oxygen atoms are bonded through a covalent bond in a carbon dioxide molecule. Here, the carbon atom forms a double bond with both the oxygen atoms i.e. the molecule has sp2 hybridization with one σ and one π bond. The bond length of both the carbon-oxygen bonds in the carbon dioxide molecule is around 116 pm.
Also, as oxygen is more electronegative than carbon there is a slight negative charge on both oxygen atoms while a slight positive charge develops on the carbon atom. However, even after the electronegativity difference the carbon dioxide molecule in itself is non-polar. This is because the carbon dioxide molecule has a linear structure indicating that the oxygen atoms are arranged at 180° from each other. They cancel out the charges and the net dipole moment of the molecule remains zero. The structure of the carbon dioxide molecule is given below:
For detailed information, read out CO2 Lewis Structure, Geometry, Hybridization, and Polarity. CO2 Ionic or Covalent
Why is CH4 Organic while CO2 is not?
Methane is an organic compound which forms a major constituent of both natural gas as well as petroleum. It is the simplest hydrocarbon that belongs to the homologous series, alkanes. It is succeeded by similar compounds viz. ethane, propane, etc. As discussed in the previous sections organic compounds are those that contain one or more carbon atoms along with hydrogen.
In a methane molecule, on carbon atom is bonded with four hydrogen atoms indicating that it is an organic compound. Also, as discussed earlier carbon dioxide is an inorganic compound as it does not contain any carbon-hydrogen bond. CH4 Lewis Structure, Geometry, Hybridization, and Polarity CH4 Intermolecular forces Is CH4 Ionic or Covalent
Properties of Organic Compounds
The organic compounds are characterized by the presence of carbon with the occasional presence of hydrogen, oxygen, or other related elements. There is a separate branch of chemistry that deals with these compounds, it is known as organic chemistry. Some of the important properties of organic compounds are listed below: • Most organic compounds have complex structures such as nucleic acids, proteins, etc., and also have high molecular weight. • A number of functional groups are known to bond with the organic compounds such as cyanide (-CN), carboxylic acid (-COOH), etc. The properties of an organic compound are decided by the functional group attached to that compound. • Most organic compounds are insoluble in water. However, they tend to dissolve in organic solvents such as benzene, toluene, etc. • Organic compounds comprise about 90% of the total compounds known to exist in the universe. • Most organic compounds are known to be volatile and combustible in nature. • The organic compounds usually have high melting and boiling points. • The rate of a chemical reaction involving an organic compound is usually slower.
Conclusion
Carbon dioxide is an inorganic compound as it does not have any hydrogen atom attached to the carbon atom. The organic compounds usually consist of the carbon atom attached with hydrogen, oxygen, nitrogen, or similar elements. However, the exceptions are also known to occur such as carbon dioxide. The carbon atom in the carbon dioxide molecule is bonded with the oxygen atom through a covalent bond and has sp2 hybridization. A double bond is present between carbon and both oxygen atoms. Methane is an organic compound as it consists of a carbon atom bonded with four hydrogen atoms while a carbon dioxide molecule does not have any hydrogen atom bonded with carbon and is, therefore, an inorganic compound. Happy learning!!