Maurice Meslans, a French pharmacist and chemist was the first person to obtain Fluoroform by reacting iodoform and dry silver fluoride in 1894. However, it is industrially produced by reacting Chloroform (CHCl3) and Hydrogen fluoride (HF) and as a by-product in or precursor to the manufacture of chemicals like Teflon. Fluoroform is a potent greenhouse gas but does not responsible for the depletion of the ozone layer. It has multiple applications such as refrigerants, fire suppressants, polymer intermediates, and more. In this article, we will discuss some of the most searched questions related to CHF3 including its Lewis dot structure, Geometry, Hybridization, and Polarity.
Lewis Dot Structure of CHF3
Lewis dot structure represents valence electrons of atoms of molecules either as lone pairs or within bonds. Lewis dot structure of CHF3 contains one central carbon atom, three fluorine atoms, and one hydrogen atom as terminal atoms.
Steps to draw Lewis Dot Structure of CHF3
Step 1: Count the total number of valence electrons in CHF3: Trifluoromethane contains four atoms – one Carbon atom, one Hydrogen atom, and three Fluorine atoms. To know the valence electrons of the atom (up to atomic number 20), remember its periodic table group number. For example, Hydrogen belongs to the 1st Group of the Periodic table so its valence electron is 1 Carbon belongs to the 14th Group of Periodic table so, its valence electrons are 4 Fluorine belongs to the 17th Group of Periodic table so, its valence electrons are 7 Total number of valence electrons available in CHF3 = 4 + 1 + 7 (3) = 26 Step 2: Choose the central atom for Lewis dot structure: The least electronegative atom of the molecule (except Hydrogen) can be represented as a central atom in Lewis dot structure. In the case of CHF3, Carbon is less electronegative than Fluorine because electronegativity increases from going left to right in the Periodic table so put carbon as central atom attached with one hydrogen and three fluorine atoms as surrounding atoms. Step 3: Connect outer atoms with central atom via single bond: Draw a skeleton molecule of CHF3 by connecting all outer atoms with a centrally placed Carbon atom via a single bond. So the total number of bonds formed in this structure is four.
Every single bond represents sharing of two electrons so, the number of valence electrons used in this structure is 4 ×2 = 8 electrons. Number of valence electrons left: 26 – 8 = 18 valence electrons Step 4: Arranging remaining valence electrons to complete octet: The octet rule states that an atom must have eight electrons in its outermost shell to become stable like Noble gases. Start arranging of remaining valence electrons from outer atom to Central atom to complete the octet. Hydrogen atom already shared two electrons via a single bond. In this way, its outermost shell gets filled. So, there is no need to put any electron around the Hydrogen atom Each Fluorine atom also shared two electrons via a single bond. In this way, it requires only 6 more electrons to complete its octet so put 6 electrons around each Fluorine atom Carbon already shared eight electrons via four single bonds. In this way, its outermost shell gets filled. So, there is no need to put any electron around the Carbon atom
Step 5: Calculate the formal charge on each atom to check stability: Formal charge determines the stability of the Lewis dot structure. Lesser the formal charge, the Higher the stability of the structure. Formula to calculate Formula Charge: Formal charge = Valence electrons in a neutral atom – lone pair electrons – 1/2 bonded pair electrons Formal charge on Carbon atom: Valence electrons of Carbon = 4 Lone pair electrons on Carbon = 0 Bonded pair electrons around Carbon (represented as a single bond) = 8 Formal charge on Carbon atom: 4 – 0 – 8/2 = 0 Formal charge on Hydrogen atom: Valence electron of Hydrogen = 1 Lone pair electrons on Hydrogen = 0 Bonded pair electrons around Hydrogen (represented as a single bond) = 2 Formal charge on Hydrogen atom: 1 – 0 – 2/2 = 0 Formal Charge on each Fluorine atom: Valence electron of Fluorine = 7 Lone pair electrons on Fluorine (represented as dots) = 6 Bonded pair electrons around Fluorine (represented as a single bond) = 2 Formal charge on each Fluorine atom: 7 – 6 – 2/2 = 0 In this structure, all atoms have a Zero formal charge so, this is the stable Lewis dot structure for CHF3.
CHF3 Geometry
Valence electron pair repulsion (VSEPR) is a model to predict the geometry of the molecule. Its main idea is molecule will take up its geometry according to the repulsion of electron pairs in the valence shells. Electrons always tend to align themselves in a way in which the distance between them remains maximum and the repulsion between them remains minimum to make the arrangement stable. The order of repulsive interaction between valence shell electrons is as follows: Lone pair (lp) – Lone pair (lp) ˃ Lone pair (lp) – Bond pair (bp) ˃ Bond pair (bp) – Bond pair (bp)
Steps to Determine the Geometry of CHF3 using VSEPR theory
• Revise Lewis structure of CHF3 • Count number of bonding electrons and lone pair electrons around the central atom • Predict the geometry according to its molecular type or VSEPR notation (expressed as ABnEm, where A represents Central atom, Bn represents the number of bonding pairs and Em represents the number of lone pairs). Note: multiple bonds like double or triple bond count as only one bonding pair In the Lewis structure of CHF3, carbon is the central atom and it has four single bonds (4 electrons) and zero lone pairs. So its VSEPR notation becomes AB4Eo or AB4. According to the following VSEPR table, the geometry of CHF3 is tetrahedral with a bond angle of 109.5⁰. Table 1 shows geometries of molecules without lone pair on the central atom:
Table 2 shows geometries of molecules with one or more lone pairs on the central atom
CHF3 Hybridization
In chemistry, Hybridization is the concept of redistribution of the energy of atomic orbitals to form a new hybrid orbital. Some of the key features of hybridization include • Atomic orbitals with equal energies participate in hybridization • The number of hybrid orbitals formed is equal to the number of participating atomic orbitals • Hybridization occurs only during the formation of the bond • It can be of several types like sp, sp2, sp3, sp3d, sp3d2 based on the number of participating atomic orbitals The number of participating orbitals can be calculated as • ½ (number of monovalent atoms attached to central atom + number of valence electrons in a neutral central atom) OR • Number of sigma bond of central atom + lone pairs on the central atom
Hybridization of CHF3
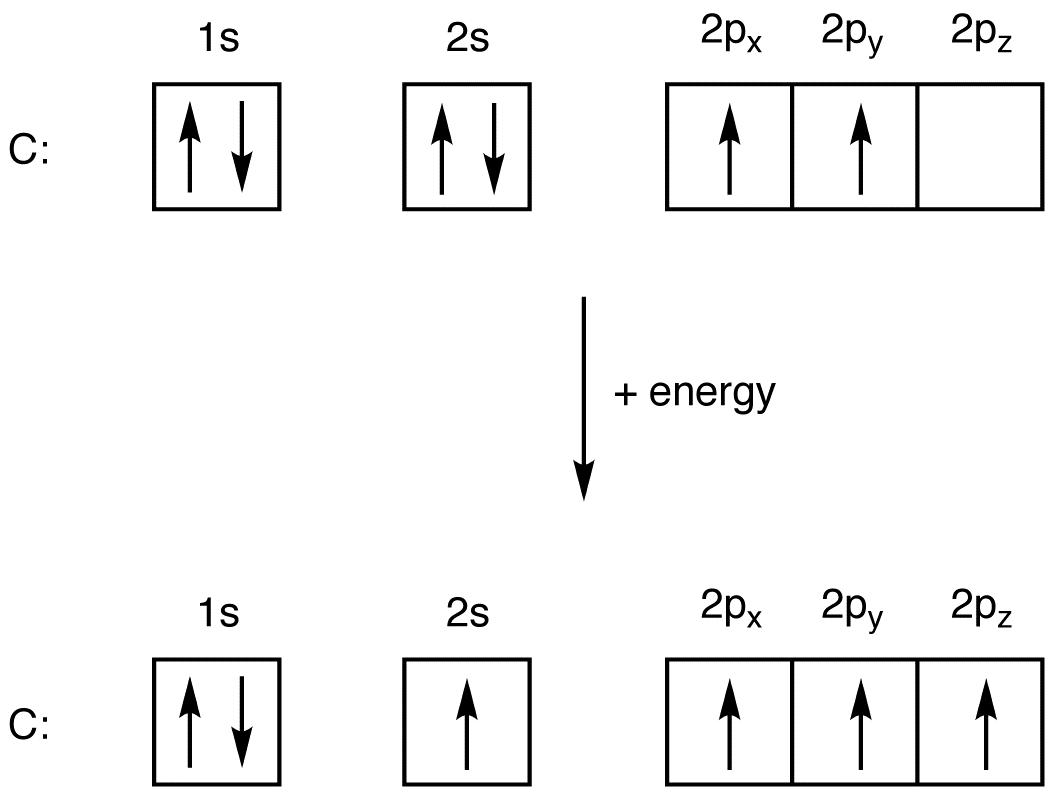
In CHF3, the number of monovalent atoms attached to the central atom = 4 Number of valence electrons in a neutral central atom = 4 By above formula, number of participating orbitals are: ½ (4 + 4) = 4 = sp3 Hybridization In other words, it can be understood as the atomic number of Carbon: 6 Electronic configuration = 1s2 2s2 2px2 2py02pz0
When Carbon gets excited, its electron from 2s orbital moves to 2p orbital (2s and 2p have similar energy levels). It’s one s and three p orbital mixed up to form four sp3 hybrid orbitals.
The atomic number of Hydrogen = 1 Electronic Configuration of hydrogen = 1s1 The atomic number of Fluorine = 9 Electronic Configuration of Fluorine = 1s2 2s2 2px22py22pz1 Hydrogen and fluorine have one unpaired electron in their 1s and 2p orbital respectively and these unpaired electrons make a bond with each sp3 hybridized orbitals.
CHF3 Polarity
Fluorine is more electronegative than Carbon so in the C-F bond, the former pulls the electron towards itself. Whereas Carbon is more electronegative than Hydrogen, in the C-H bond, the former pulls electrons towards itself. Being the most electronegative, fluorine act as the negative end of the molecule whereas the hydrogen end of the molecule act as the positive terminal of the molecule. Hence, CHF3 has a non-zero dipole moment. CHF3 is a polar molecule.
The Polarity of CHF3 Depends on
Electronegativity
Polarity increases when the difference of electronegativity between atoms increases. In the case of CHF3, Fluorine has an electronegativity of 3.98 whereas Carbon has an electronegativity of 2.6. The difference in electronegativity between Fluorine and Carbon leads to asymmetric distribution of charges around the atom.
Geometry or shape of the molecule
Asymmetric structures are generally polar in nature. CHF3 has tetrahedral geometry and is asymmetric in nature so it is considered to be polar. Related Topics CH4 Lewis Structure CCl4 Lewis Structure HF Lewis Structure COF2 Lewis Structure NO2F Lewis Structure BrCl Lewis Structure ICl3 Lewis Structure SiCl2Br2 Lewis Structure H2O2 Lewis Structure ClO3 Lewis Structure Al2O3 Lewis Structure
Conclusion
Fluoroform is a haloform with the chemical formula CHF3. It is a potent greenhouse gas but does not responsible for ozone depletion Lewis structure of CHF3 has 26 valence electrons in which 18 are lone pair electrons and 8 are bond pair electrons. CHF3 has four bond pairs and zero lone pairs so according to VSEPR theory, it has tetrahedral geometry with a bond angle of 109.5 degrees The hybridization of CHF3 is sp3 in which one s and three p orbitals hybridized together to form four sp3 hybrid orbitals CHF3 is a polar molecule due to the difference in electronegativity of atoms as well as an asymmetrical structure. The dipole moment of CHF3 is 1.8 D. Happy Reading!!