In this article, we will understand the concepts of prediction of Lewis structure, geometry, hybridization, and polarity of a given compound.
Lewis Structure
Lewis Structure is a 2-D representation that depicts the arrangement of atoms and valence shell electrons on those atoms in a compound. Many attempts were made to explain the formation of the chemical bond. Lewis was among the first ones to explain. He postulated that only valence electrons take part in bonding, and hence in his representation, only valence electrons are considered. In this method, valence shell electrons are represented by dots on the chemical symbol of elements. There can be a possibility of more than one Lewis structure for some compounds. To find the most accurate and lowest energy structure, we must satisfy the octet rule and formal charges on all the constituent atoms.
Octet Rule
Noble gases are considered the least reactive and most stable in the periodic table. Every element forms a bond to attain a noble gas-like configuration. Noble gases have eight electrons in their valence shell, except He. Hence all main group elements prefer to have a fully filled configuration by having a net of 8 electrons in their valence shell after bonding. This preference for 8 electrons gives rise to the octet rule.
Formal Charge
It is a theoretical concept. It compares the number of valence electrons in an isolated neutral atom with the number of valence electrons in the bonded form in a molecule. It is calculated using the given formula- Formal charge= (number of electrons in an isolated neutral atom)- (number of nonbonding electrons on an atom in the compound)- 0.5*(number of electrons shared in bonds by atom)
Steps to draw Lewis Structure of AlCl3
Step 1. Count the total number of valence shell electrons on the compound. Before drawing the structure, we need to know the number of valence shell electrons on all constituent atoms and their sum. Step 2. Draw the lewis dot structure for elements. We draw the Lewis structure of elements by arranging the valence shell electrons around the element’s chemical symbol. The chemical symbols for aluminum and chlorine are Al and Cl, respectively. The Lewis dot structure for Al and Cl are as follows-
Step 3. Choose a suitable central atom for the compound. The central atom is supposed to be the least electronegative one out of the constituent atoms as the central atom is supposed to share its electron density with all other atoms. Thus, Al is the central atom for this compound. Step 4. Draw a skeletal diagram. In this step, we have to arrange the side atoms and central atoms suitably.
Step 5. Arrange the valence electrons around the elemental symbols. The total valence shell electrons (calculated in step 1) are placed according to a predicted bond formation. The blue dots represent electrons from Al, and black dots represent electrons from Cl.
Step 6. Complete the octet of atoms by forming bonds. Each Cl has seven valence electrons in the isolated state. They share one electron with Al to have a fully filled valence shell configuration. Al has three valence electrons in the isolated state. It shares one electron from all Cl-atoms, and still, the octet does not go to completion. There is no alternate lewis structure in which the octet is complete. It is always short of two electrons, and hence it is an electron-deficient compound.
Step 7. Calculate the formal charge on all atoms. The net charge on this compound is zero. Therefore, the sum of formal charge on three atoms should come out to be zero. Thus, the structure drawn in step 6 is the best Lewis structure for AlCl3. Here is a video attached regarding steps to draw the lewis structure of AlCl3. You can go through it.
AlCl3 Geometry
Lewis structure does not attempt to predict molecular geometry and shape. For the geometry, we have another theory called VSEPR theory. Molecular geometry is the 3D arrangement of atoms in a molecule. VSEPR theory stands for valence shell electron pair repulsion theory. According to VSEPR theory- • The valence electron pairs repel each other, and this leads to instability. • To make the arrangement of the electrons stable, the repulsions between them have to be decreased. • As a result, electrons align themselves so that the repulsion is the least, and the distance between them is maximum. • The stable arrangement of the valence electron pairs of atoms helps determine the molecular geometry. Valence shell electrons involved in bonding are known as bonding pairs of electrons (bp), and those valence shell electrons that are not involved in bonding are termed as lone pairs of electrons (lp). You must also read out the article I wrote on is AlCl3 ionic or covalent.
How to Predict Geometry of AlCl3 Using VSEPR
- Count the number of valence shell electrons on the central atom and let it be equal to A (arbitrary variable). In the case of AlCl3, the central atom is Al. Al has 3 valence electrons. (Shown in step1 of drawing lewis structure) A=3
- Count the number of side atoms and let it be equal to B (arbitrary variable). In AlCl3, there are three side atoms chlorine) and B=3
- If the compound is charged, subtract the charge from B for the positively charged compound and add the charge to B for the negatively charged compound. This step can be avoided for neutral compounds. In AlCl3, there is no contribution of charge and B=3 only.
- Add the contribution of side atoms and charge to the contribution of the central atom, i.e., A+B. For AlCl3, A+B=6
- Divide A+B by 2 to find total electron pairs affecting the shape. For AlCl3, there are 3 electron pairs.
- Divide the total electron pairs as bonding and non-bonding. The bonding electron pair is equal to the number of side atoms. For AlCl3, there are three side atoms. Thus, there are three bonding pairs of electrons and zero nonbonding pairs of electrons. Using this information, we can predict geometry and shape using the following table. The electron geometry and shape of AlCl3 are trigonal planar. The geometry and shape are the same for compounds with zero lone pairs.
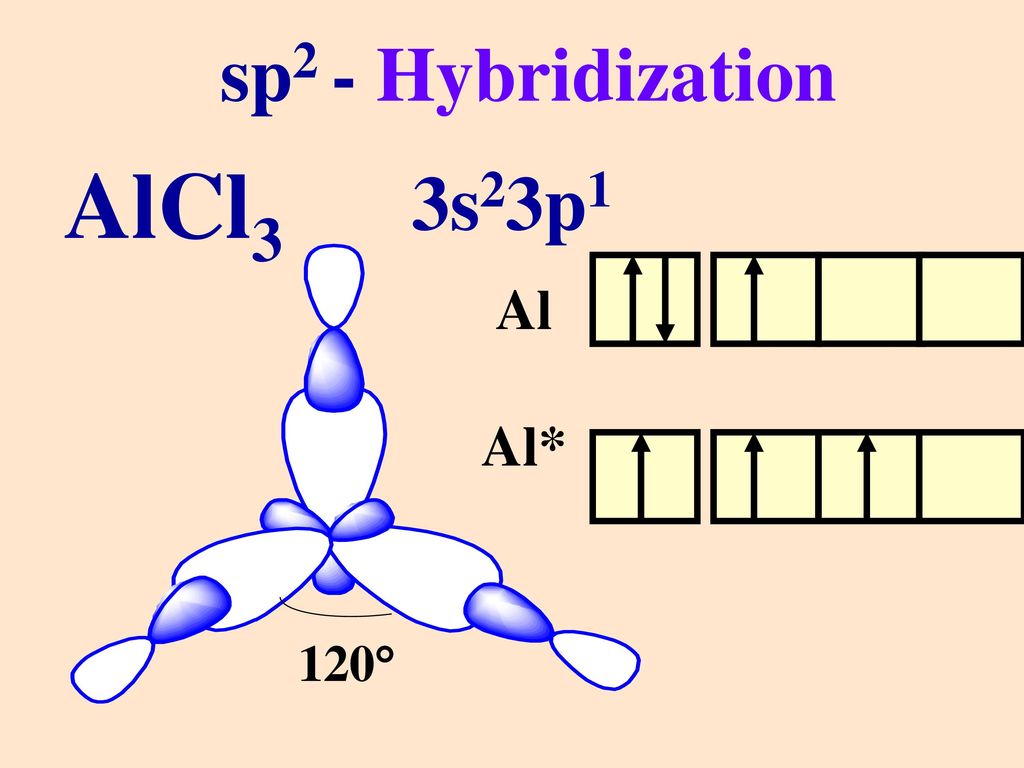
AlCl3 Hybridization
In some polyatomic compounds like methane, we need a new concept to explain the bonding. This concept is hybridization. Hybridization is the mixing of atomic orbitals, which are similar in energy, size, and shape, to form equivalent orbitals. It involves the redistribution of energy. There is no actual mixing; just mixing of wavefunctions takes place. For instance, one 3s and three 3p orbitals can mix to form four sp3 hybrid orbitals, but 1s and 5p cannot. In AlCl3, Al is the central atom. The ground state electronic configuration of Al is 1s2 2s2 2p6 3s2 3p1. Only valence orbitals are used in hybridization.
One electron of 3s gets promoted to 3p orbital in the excited state. Now, these 3 orbitals (one 3s and two 3p) undergo hybridization to form three sp2 orbitals, which will form bonds with the p orbital of surrounding chlorine atoms. One p orbital of Al remains vacant. Thus, AlCl3 has sp2 hybridization.
The trick for calculating the type of hybridization of AlCl3
In VSEPR theory, we calculated the total electron pairs in the last step. For AlCl3, it came out to be 3. The table below can predict hybridization using total electron pairs or steric numbers. Steric number = number of (sigma bonds +lone pair on central atom)
Steric number for AlCl3= (3+0)=3 Hybridization comes out to be sp2 from the table.
AlCl3 Polarity
The polarity of a compound can be determined by the presence or absence of a net dipole moment. The net dipole moment of a compound depends on- • Dipole moment of the bond • The difference in electronegativity of atoms constituting the bonds • Geometry/Symmetry of compound Dipole moment is a vector quantity. In AlCl3, there is only one type of bond, “Al-Cl.” The electronegativity of Al and Cl are 1.61 and 3.16, respectively. The difference comes out to be 1.55. Hence, we can say that the bonds are polar and the bond dipole moment is non-zero. Polar bonds do not guarantee a polar molecule. The vector’s sum of the dipole moment of the three bonds comes out to be zero due to the trigonal planar shape. The three vectors are at an angle of 120°, and they cancel each other out. Hence, AlCl3 is a non-polar molecule.
Conclusion
AlCl3 is an inorganic metal halide. It is an electron-deficient compound as the octet of Al is not complete. The Lewis structure drawn in the above section is suitable for aluminum chloride. The molecular geometry and shape come out to be trigonal planar. The central atom, Al, is sp2 hybridized. Aluminum chloride is non-polar. Happy reading!